A Clear Difference
Tri-Luma Cream provides clear improvements you can see.**
77% of Tri-Luma Cream subjects experienced complete or near-complete clearing of
their facial melasma by week 8.1,2
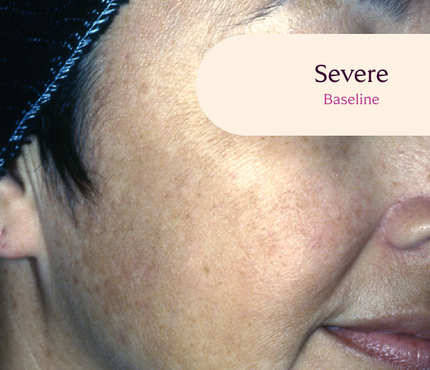
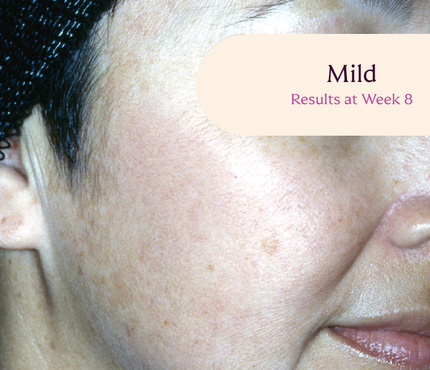
Before and After
Tri-Luma Cream provides clear improvements you can see.**
77% of Tri-Luma Cream subjects experienced complete or near-complete clearing of
their facial melasma by week 8.1,2
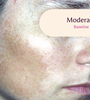
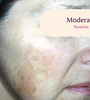
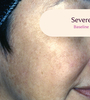
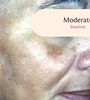




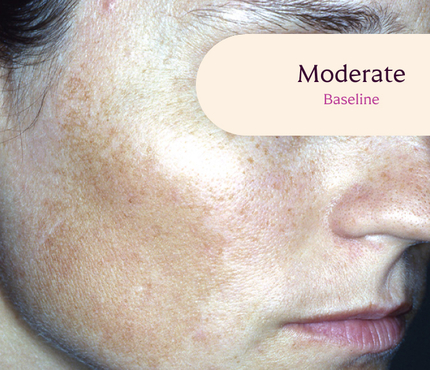
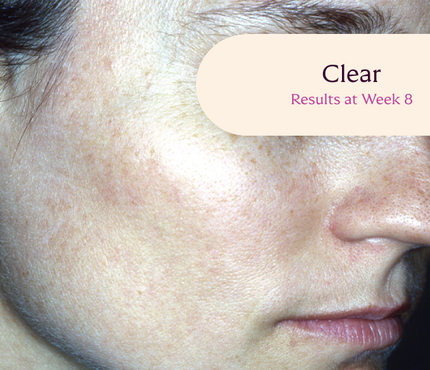
Skin type: II
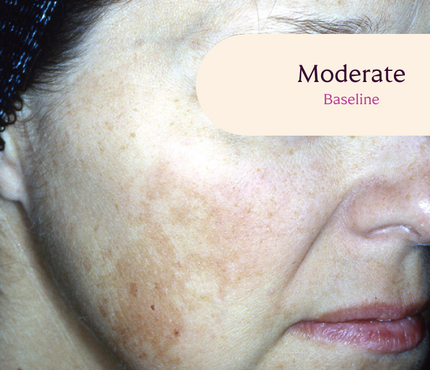
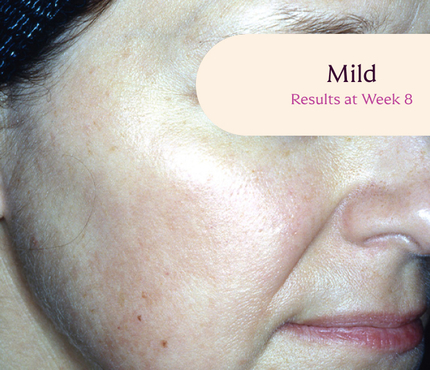
Skin type: III
Skin type: III
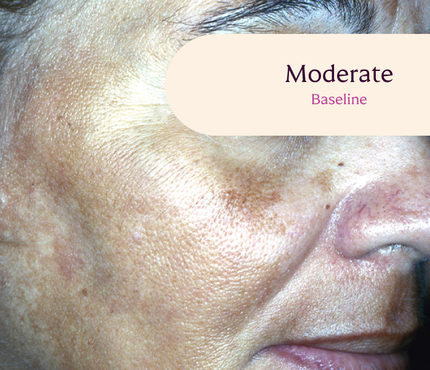
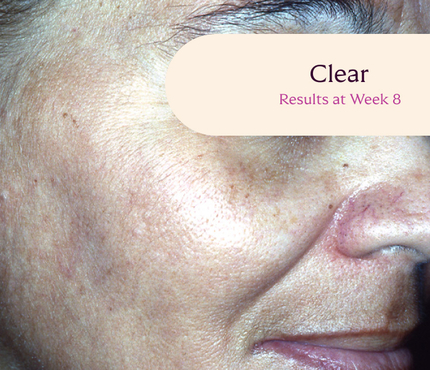
Skin type: IV
Study Design
The safety and efficacy of Tri-Luma Cream were evaluated for the treatment of moderate to severe facial melasma in two phase 3, multicenter, randomized, investigator-blind, active-control, parallel-group trials, which were identical in design. The trials were conducted in 641 subjects aged 21 to 75 years with Fitzpatrick skin types I through IV. Subjects were treated once daily at night for 8 weeks with Tri-Luma Cream (hydroquinone [HQ, 4%] + tretinoin [RA, 0.05%] + fluocinolone acetonide [FA, 0.01%]) or 1 of 3 possible combinations of 2 of the 3 active ingredients contained in the same vehicle: RA 0.05% + HQ 4%, HQ 4% + FA 0.01%, or RA 0.05% + FA 0.01%. For enrollment, subjects had to demonstrate stable facial hyperpigmentation for at least 3 months, macular lesions that were neither depressed nor atrophic, and melasma severity scores of 2 or higher. The primary efficacy endpoint was the proportion of subjects achieving complete clearing (melasma score 0) at Week 8. The secondary efficacy endpoint was the proportion of subjects achieving complete (melasma score 0) or near-complete (melasma score 1) clearing by Week 8. Both the primary and secondary efficacy endpoints were calculated from a pooled analysis from clinical study reports.
**All photographs were taken from phase 3 clinical trials. Individual results may vary.
The Highest Standard in Melasma Therapy
Tri-Luma Cream is the only FDA-approved1* topical treatment available to help treat the dark spots associated with moderate-to-severe facial melasma for the short-term (up to 8 weeks). The precise combination of 3 active ingredients works synergistically† to deliver clear results.1-7
*Tri-Luma Cream is the only approved prescription, fixed-dose combination containing hydroquinone for facial melasma.

Help Reduce Inflammation
FLUOCINOLONE ACETONIDE 0.01%
is a mild topical corticosteroid with anti-inflammatory effects. It reduces the irritative effects of the 2 other agents, and inhibits melanin synthesis by decreasing cellular metabolism.8

Even Out Skin Pigmentation
HYDROQUINONE 4%
is a depigmenting agent that interrupts the formation and synthesis of melanin to help even and lighten the skin.8

Protect Skin Long Term
TRETINOIN 0.05%
is a retinoid that may prevent oxidation of hydroquinone and improves epidermal penetration and skin cell turnover, facilitating pigment elimination and reducing the risk of skin atrophy.8
†The mechanism of action of the active ingredients in Tri-Luma Cream in the treatment of facial melasma is unknown.
Get the Facts About Facial Melasma
If you think you may have melasma, you’re not alone
as over 5 million people are affected by melasma.9 Although the exact cause is unknown, melasma may be triggered by sun exposure, pregnancy, birth control pills and hormone therapy treatments.10 While melasma can affect anyone, it is more prevalent among women with darker skin tones.9

Tri-Luma Cream may improve the appearance of facial melasma
but is not a cure and should always be used in conjunction with sun-avoidance measures.1 Protect your skin by using a broad-spectrum sunscreen with SPF of 30 or higher and by wearing protective clothing. Even a small amount of sun exposure can trigger or worsen melasma.1

The more you know about facial melasma, the better you can control its impact.
Ask your doctor for more details, and if FDA-approved Tri-Luma Cream is right for you.
Possible Side Effects of Tri-Luma Cream
Some patients may have very severe allergic reactions to Tri-Luma Cream due to the sulfites contained in the product. In some cases, these allergic reactions may result in severe asthma attacks, which can be life threatening.
While you use Tri-Luma Cream, your skin may develop mild-to-moderate redness, peeling, burning, dryness, or itching.
Tri-Luma Cream contains a corticosteroid medicine as one of its active components. The following side effects have been reported with application of corticosteroid medicines to the skin: itching, irritation, dryness, infection of the hair follicle, acne, changes in skin color, inflammation around the mouth, allergic skin reaction, skin infection, skin thinning, stretch marks and sweat problems.
In addition, Tri-Luma Cream contains a corticosteroid that may produce reversible effects on the adrenal gland called hypothalamic-pituitary-adrenal (HPA) axis suppression, so tell your doctor if you have any condition that may affect your adrenal function, such as Addison’s disease.
Some patients using Tri-Luma Cream develop dark spots on their skin, tingling, increased skin sensitivity, rash, acne, skin redness caused by a condition called rosacea, skin bumps, blisters, or tiny red lines or blood vessels showing through the skin. Tri-Luma Cream may also cause a gradual blue-black darkening of your skin; if this happens, stop using the product immediately and speak to your doctor.
If you are concerned about how your skin is reacting to Tri-Luma Cream, call your doctor.
You are also encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Stopping Treatment with Tri-Luma Cream
Stop using Tri-Luma Cream and contact your doctor if you experience:
- Severe or continued irritation, blistering, oozing, scaling, or crusting
- Severe burning or swelling of your skin
- Irritation of your eyes, nose, or mouth
- Any type of breathing problem
- Blue-black coloration on your skin where the cream was applied
If you become pregnant while taking Tri-Luma Cream, tell your doctor right away. You should discuss the chances that your baby may be harmed. Using Tri-Luma Cream early in pregnancy may be more likely to produce birth defects than using it later in pregnancy.
Additionally, Tri-Luma Cream is indicated for the short-term (up to 8 weeks) treatment of moderate-to-severe melasma of the face. It is not indicated for long-term use (more than 8 weeks) or for the maintenance of melasma symptoms.
What to Avoid While Using Tri-Luma Cream
You should avoid sunlight and ultraviolet light. Tri-Luma Cream can make your skin more likely to get sunburned or develop other unwanted effects from natural or artificial sunlight (as from a sunlamp or tanning bed). Dark skin patches may also become darker when your skin is exposed to sunlight. Any level of sun exposure can contribute to or trigger melasma—you don’t have to be sunburned to make your melasma worse.
It is important to protect your skin from the sun to help prevent further darkening of existing dark patches and the formation of new ones.
Staying out of the sun is especially important for women who take birth control pills or hormone replacement therapy, and for people who have had dark patches in the past.
Follow these guidelines regarding sun exposure when using Tri-Luma Cream:
- Use an effective sunscreen any time you are outside, even on hazy days. The sunscreen should have an SPF (sun protection factor) of 30 or more. Use sunscreen year-round on areas of the skin that are regularly exposed to sunlight, such as your face and hands. If possible, protect the treated area from sunlight exposure.
- If you spend a lot of time outside, be especially careful of sunlight. Ask your doctor what SPF level will give you the needed high level of protection. If you will be outside, wear protective clothing, including a hat.
- Do not use sunlamps or any artificial source of sunlight (such as tanning beds) while you use Tri-Luma Cream.
Skin treated with Tri-Luma Cream may be more likely to react to heat and cold than untreated skin. Your doctor can recommend ways to manage your melasma under these conditions.
While using Tri-Luma Cream, you should avoid products that may dry or irritate your skin. These may include soaps and cleansers that are rough or cause dryness; certain astringents, such as alcohol-containing products, soaps and toiletries containing alcohol, spices, or lime; or certain medicated soaps, shampoos, and hair permanent products.
Do not use any other medicines with Tri-Luma Cream unless you have consulted your doctor. The medicines and products you have used in the past may cause redness or peeling when used with Tri-Luma Cream.
Eligible Patients May Save
Galderma CAREConnect‡ Patient Savings Program makes it easy to save on Tri-Luma Cream. Simply download and present when filling your Tri-Luma Cream prescription or refill.

‡The Galderma® CAREConnectTM Program (“Program”) is brought to you by Galderma Laboratories, L.P. (“Galderma”). The Program is only available at participating pharmacies for patients with commercial insurance or patients without insurance. Patients who are enrolled in a state or federal government run or government sponsored healthcare plan can not participate in the Program. Any claim under the Program must be submitted by participating pharmacies to one of the Administrators of the Program.
The Program is subject to applicable state and federal law and is void where prohibited by law, rule or regulation. In the event a lower cost generic drug that the FDA has designated as a therapeutic equivalent product is available for one of the Galderma products covered by the Program, or if the active ingredient of a Galderma product is available at a lower cost without a prescription, this offer will become void in California with respect to the Galderma product
Important Safety Information
Indication: TRI-LUMA® (fluocinolone acetonide 0.01%, hydroquinone 4%, tretinoin 0.05%) Cream is indicated for the short-term (up to 8 weeks) treatment of moderate to severe melasma of the face in the presence of measures for sun avoidance, including the use of sunscreens. Adverse Events: In the controlled clinical trials, the most frequently reported events were redness, peeling, burning, dryness, and itching at the site of application. Warnings/Precautions: TRI-LUMA Cream contains sulfites which may cause severe, life-threatening allergic reactions in people allergic to sulfites. TRI-LUMA Cream contains hydroquinone, which may cause a gradual blue-black darkening of the skin. If you are pregnant, nursing or trying to become pregnant you should not use TRI-LUMA Cream. Safety and efficacy have not been established in individuals with darker skin. Reversible HPA axis (adrenal function) suppression may result from exposure to the topical corticosteroid, fluocinolone acetonide, so discontinue use if signs and symptoms of this condition occur. Avoid products that may dry or irritate the skin, such as abrasive cleansers, scrubs, or skin-peeling agents. Exposure to sunlight, sunlamps, or UV light and extreme heat, wind, or cold should be avoided. If exposure cannot be avoided, sunscreen products [SPF 30 or more] and protective apparel should be used.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
- TRI-LUMA (fluocinolone acetonide 0.01%, hydroquinone 4%, tretinoin 0.05%) Cream [Prescribing Information]. Dallas, TX: Galderma Laboratories, L.P.; 2014.
- Taylor SC, Torok H, Jones T, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72(1):67-72.
- Vashi NA, Kundu RV. Facial hyperpigmentation: causes and treatment. Br J Dermatol. 2013 Oct;169 Suppl 3:41-56. doi: 10.1111/bjd.12536. PMID: 24098900.
- Ogbechie-Godec OA, ElbuluK N. Melasma: an up-to-date comprehensive review. Dermalol Ther (Heidelb). 2017;7(3):305-318. doi:10.10071/s13555-017-0194-1.
- “Melasma.” American Academy of Dermatology Public Resource Center. TRI-LUMA (fluocinolone acetonide 0.01%, hydroquinone 4%, tretinoin 0.05%) Cream.
- Grimes P, Watson J. Treating epidermal melasma with a 4% hydroquinone skin care system plus tretinoin cream 0.025%. Cutis. 2013;91(1):47-54.
- Ference JD, Last AR. Choosingtopical corticosteroids. Am Fam Physician. 2009;79(2):135-140. 8. Kagha K, Fabi S, Goldman MP. Melasma’s impact on quality of life.J Drugs Dermatol. 2020;19(2):184-187. doi:10.36849/ JDD.2020.4663.
- Rendon et al. Treatment of melasma. J Am Acad Dermatol. May 2006. doi: 10.1016/j.jaad.2005.12.039.
- Kagha K, Fabi S, Goldman MP. Melasma’s impact on quality of life.J Drugs Dermatol. 2020;19(2):184-187. doi:10.36849/ JDD.2020.4663.
- "Melasma," my.clevelandclinic.org, accessed June 14, 2022.
